Improving Hospital Care for ICU and Diabetic Patients using Artificial Intelligence
How JKI helped a healthcare startup navigate the FDA validation process and speed a life-saving device to market.
By Philippe Guerit and Henry Sillin, JKI
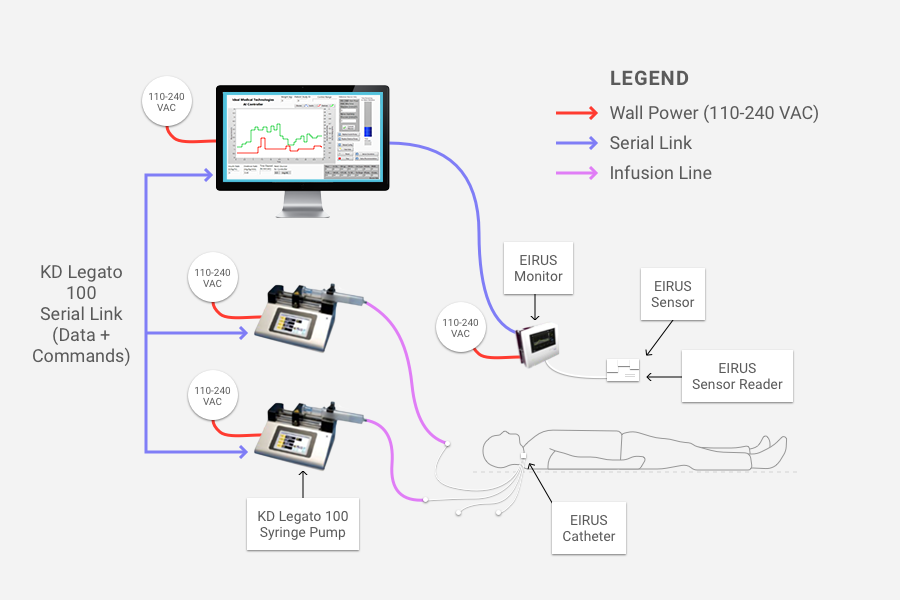
In hospital intensive care units (ICUs), controlling patients’ blood glucose levels is critical. The stress caused by critical illness can lead to large fluctuations in those levels, which can increase mortality rates. Ideal Medical Technologies, a startup founded by a medical doctor and his engineer son, has created a solution that acts as an artificial pancreas. This artificial pancreas continuously monitors the glucose level and uses Artificial Intelligence (AI) based software to keep the glucose level in a safe range through frequent adjustments of insulin and glucose infusions.
Need help navigating the FDA validation processfor your LabVIEW application?
|
The product, called FUSION, has the potential to save 100,000 lives and $5 billion a year in healthcare costs in the United States, according to Dr. Leon DeJournett, Ideal Medical Technologies founder and Chief Medical Officer (CMO). Traditional manual methods of controlling glucose are labor intensive, taking up to two hours a day per patient. Through use of an AI control technique, FUSION takes only 20 minutes or less per day, dramatically improving nursing efficiency. What’s more, it’s safer than current alternatives and has the potential to decrease complications, mortality rates and length of hospital stays.
FUSION is so significant that it has been designated as a “Breakthrough Medical Device” by the U.S. Food and Drug Administration (FDA). This designation for products that provide a more effective treatment of life-threatening diseases or conditions than products currently on the market, prioritizes and speeds up the FDA approval process. The FDA has determined that FUSION’s AI based glucose control software is a Class II medical device, a category of products ranging from moderate to high risk to the patient. Wheelchairs and IVs, for example, fall into this category.
After designing Fusion using LabVIEW, Ideal sought a partner to help it develop the testing and validation of software required for the FDA’s investigational device exemption application. The company chose JKI because of its renown as a LabVIEW consultant, with many products and open source libraries, and its extensive experience in the life sciences.
Challenge 1: Understanding the product
Fusion consists of three components:
- A continuous glucose monitor designed specifically for the ICU. This component, supplied by Maquet Critical Care which is a Swedish medical device maker, tracks the patient’s glucose levels.
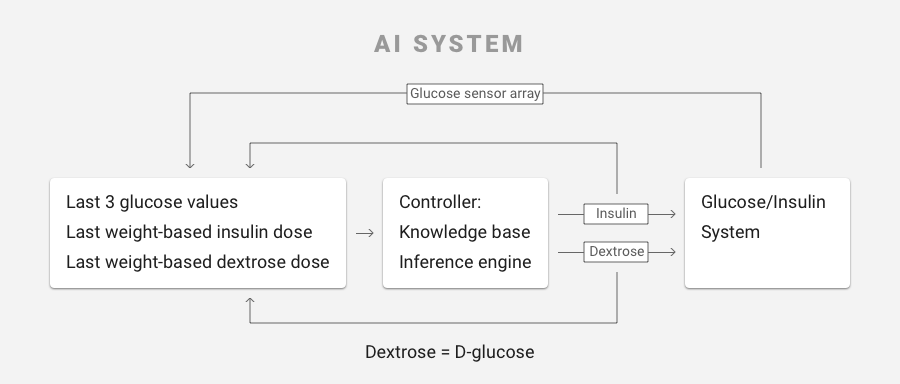
- Artificial intelligence-based software, written by Ideal, that takes inputs such as patient weight and current glucose level, then applies algorithms that produce the correct infusion rates of insulin and glucose needed to maintain safe glucose levels. The algorithms mimic the functions of the pancreas (which produces insulin) and the liver (which produces glucose). The software operates like a smart prescription system and in the prototype of the FUSION system runs on a laptop connected to the glucose monitor and the infusion pumps.
- Intravenous infusion pumps. This component infuses the doses of insulin and glucose into the patient.
JKI worked with Ideal to understand how the product meets medical needs, how the components work together, and how the AI software works in particular. The code was written by Jeremy DeJournett, Ideal’s CTO and Dr. DeJournett’s son, for the initial minimum viable product (MVP) and proof of concept phases. JKI brought a high level of code expertise to the project, helping to improve the code and its traceability, as well as mitigating risk.
Challenge 2: Developing the validation framework
As part of the FDA application, Ideal had to provide validation data showing that the AI-based software and the user interface worked as described. The data needed to demonstrate that insulin and glucose infusions would always be within the safe range in which FUSION is designed to operate. Such validation is of vital importance in Class II medical devices.
This required that JKI understand the intricacies of how the AI software processed various inputs. Through the glucose monitor, the software receives patient glucose levels at regular intervals of five to 10 minutes. If the levels are not in the range that the doctor has prescribed, the software applies knowledge-based rules, using various values to recalculate the appropriate infusion rates of insulin and glucose needed to keep the patient’s glucose in the prescribed range, then adjusts these infusion rates every 5 or 10 minutes. The inputs to the AI based glucose control software include patient weight, desired glucose range, current absolute glucose value, the glucose rate of change over the previous two cycles, and the current intravenous infusion rates of insulin and glucose. If the patient’s glucose level is dangerously low, the AI software may deliver a quick bolus of glucose to quickly raise it to a safer range, then readjust the insulin and glucose infusion rates to maintain the glucose level in the desired range. Alternately, if the measurements show levels are too high, the system may give a bolus of insulin and readjust the infusion rates of both insulin and glucose in order to produce a gradual decline of the level towards the desired range.
While the objective may be simple, achieving it is complex. The algorithms evaluate patient conditions into one of several hundred unique scenarios, all of which required validation. The validation had to show likely points of failure, and how Ideal mitigated any risk to the patient in the event of such failure. In addition, as the AI code is tweaked in further development and trials, it must be validated again. That made it a potentially huge, time-consuming job.
JKI devised an elegant solution. Rather than having to manually validate each possible outcome, JKI developed a validation framework. The framework automates data collection and logging, enabling Ideal to quickly and efficiently validate the code, even as it continues to fine-tune it.
“Now, with JKI's framework, we can run all these tests with one click, and instantly know whether our assumptions will hold, no matter what state the code is in.”
- Jeremy DeJournett, CTO, Ideal Medical
“It also was great having a fresh set of eyes that understand LabVIEW and could show us where the software or the documentation might be lacking.”
Challenge 3: Aggressive timetable
The designation of the product as a “Breakthrough Medical Device” meant there was an aggressive schedule for developing the validation framework. Not only was it important to get the FDA application and accompanying data submitted as soon as possible, but getting the product into trials and on the market quickly could save lives. JKI had the framework completed in four months.
Project status
Ideal is pleased with the outcome of its partnership with JKI. The validation framework, which automates and makes the process so much more efficient, is a success and a key element in the process of achieving FDA approval and moving to human trials. Noting that he was new to software development, CTO DeJournett found JKI’s expertise and experience in LabVIEW especially valuable. “They helped me revise the code so things could be done in a more traceable way and in ways that mitigate risks,” he says.
CMO DeJournett agrees. “We didn’t know what we didn’t know. JKI helped us with the code, helped us get our documentation in order, and helped ensure the software met the FDA’s standards.”



